
Gain’s lead drug candidate GT-02287 is a best-in-class small molecule, for the treatment of GBA-Parkinson’s disease and other neurodegenerative diseases. GT-02287 is currently being evaluated in a Phase I clinical study.

By leveraging AI-supported 3D structural biology and supercomputer-powered proprietary physics-based models, Gain’s Magellan™ platform is able to identify novel allosteric binding sites on disease-implicated proteins and exploit their untapped opportunities, by pinpointing pockets that cannot be found or drugged with current technologies.
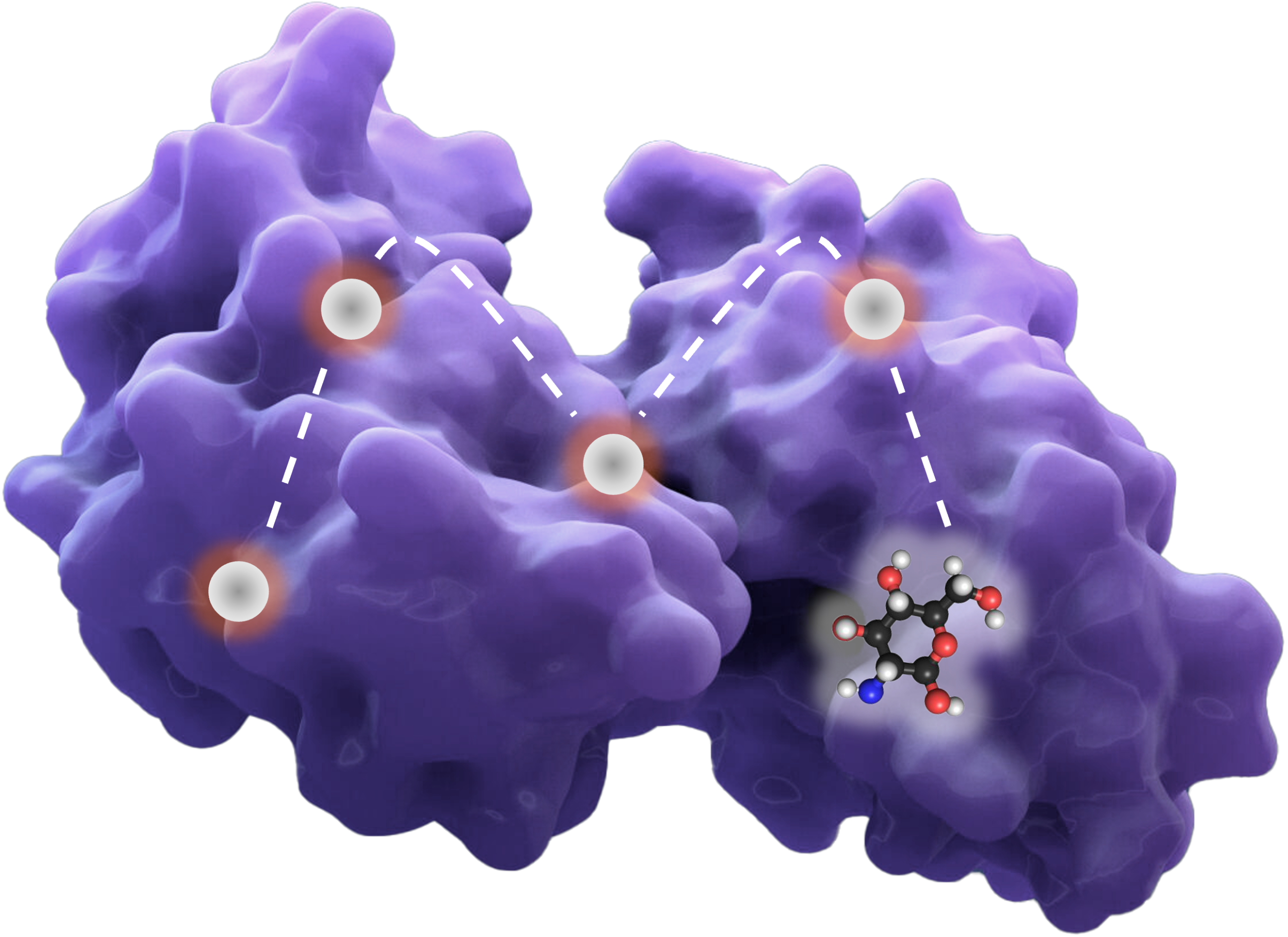

Our next generation allosteric small molecule therapeutics can modulate a protein to restore or disrupt function, not just through inhibition or activation, but also through stabilization, destabilization and degradation, based on disease pathology.

The Company has received approval to begin enrollment of the Phase 1b clinical trial in Australia Phase 1b clinical trial will assess safety and tolerability …
The Company has received approval to begin enrollment of the Phase 1b clinical trial in Australia Phase 1b clinical trial will assess safety and tolerability …
Presentation to Feature Lead Drug Candidate GT-02287 and Magellan Drug Discovery Platform BETHESDA, Md., Dec. 11, 2024 (GLOBE NEWSWIRE) — Gain Therapeutics, Inc. (Nasdaq: GANX) …