
- Details
- Written by: Developer SM
- Hits: 374
Idiopathic Parkinson’s Disease
Parkinson’s disease (PD) is caused by the degeneration of dopaminergic neurons and the resulting reduction of dopamine levels in the part of the brain that controls movement, which leads to the symptoms of PD, including tremors of the hands, arms, legs and jaw, muscle rigidity or stiffness of the limbs, gradual loss of spontaneous and automatic movement, loss of balance, and in some cases, cognitive decline.
In idiopathic PD, there is no apparent environmental or genetic cause. However, studies have shown that the activity of the lysosomal enzyme glucocerebrosidase (GCase) is significantly reduced in this patient population.
In GBA1 Parkinson’s disease (GBA1-PD), the disease is associated with mutations in the GBA1 gene, found in up to 15% of patients with Parkinson’s disease and making it the primary genetic risk factor. The mutation causes GCase misfolding and dysfunction, reducing its activity in the brain and leading to the subsequent accumulation of α-synuclein and degeneration of dopaminergic neurons. Patients with GBA1-PD tend to have earlier onset and faster symptom progression than idiopathic PD as well as a higher rate of cognitive decline associated with disease progression.
- Details
- Written by: Developer SM
- Hits: 399
Alzheimer’s Disease
Dementia with Lewy bodies (DLB) and Alzheimer’s disease are both dementia types that lead to decline in memory, thinking and behavior. In DLB, deposits of the protein α-synuclein, called Lewy bodies, aggregate in neuronal cells. GT-02287 has been shown to reduce α-synuclein aggregation in preclinical models.
In Alzheimer’s, the main pathophysiology involves the development of amyloid plaques and neurofibrillary tangles. Lysosomal dysfunction has been shown to play a role in aggregation of Tau and development of amyloid plaques. GT-02287 has been shown to reduce tau hyperphosphorylation and neuronal death in response to aggregated amyloid-beta in preclinical models.
- Details
- Written by: Developer SM
- Hits: 528
Gaucher disease is the most common lysosomal storage disease and is caused by mutations in the GBA1 gene, which encodes the glucocerebrosidase (GCase) enzyme. The mutation causes misfolding and dysfunction of the lysosomal enzyme glucocerebrosidase (GCase), which leads to the toxic buildup of fat in a variety of organs and tissues such as the liver, spleen, bones and central nervous system.
Symptoms vary by patient, but can include enlargement of organs, easy bruising, seizures and extreme fatigue. The brain abnormalities manifest gradually as seizures, cognitive deficits, poor coordination and eye movement abnormalities. Current treatments of Gaucher disease cannot penetrate the blood-brain barrier, and as such, cannot treat the neurological symptoms of Gaucher disease.
Gain Therapeutics’ lead drug candidate, GT-02287 is an allosteric small molecule regulator of GCase that can cross the blood-brain barrier. In preclinical models, GT-02287 has been shown to increase GCase activity and to decrease toxic fat accumulation in the cells of the central nervous system, and may provide a treatment option for neuronopathic Gaucher disease.
- Details
- Written by: Developer SM
- Hits: 419
GM1 Gangliosidosis is a hereditary, progressive disease mostly impacting neurons in the brain and spinal cord, caused by mutations in GLB1, the gene that encodes the beta-galactosidase (GLB) enzyme. These mutations result in the misfolding and subsequent dysfunction of GLB, which leads to the toxic substrate accumulation of GM1 ganglioside in organs and tissues. Symptoms include hypotonia, enlargement of spleen and liver, seizures and visual impairment. Gain has developed novel small molecule allosteric regulators of GLB that have been shown to significantly restore GLB function and reduce intracellular toxic substrates in a preclinical model of GM1 gangliosidosis.
- Details
- Written by: Developer SM
- Hits: 401
Krabbe disease is a severe neurodegenerative disorder caused by mutations in GALC, the gene that encodes the galactosylceramidase (GALC) enzyme. These mutations cause GALC misfolding and dysfunction, which leads to the toxic buildup up fats in the central nervous system and ultimately leads to the demyelination and death of neurons. Symptoms of Krabbe disease include muscle weakness, inability to move, seizures and decline in mental ability. There is no cure for this disease and most patients will die before the age of two. Gain is developing allosteric regulators to restore GALC function and potentially limit neuronal cell death, potentially providing the first treatment option to patients with this high unmet need.
- Details
- Written by: Developer SM
- Hits: 428
Alpha-1 antitrypsin (AAT) deficiency is an inherited disorder that can cause disease in the lung and liver. Symptoms include inability to breathe, recurring respiratory infections and fatigue as well as liver cirrhosis, leaving patients at risk for development hepatocellular carcinoma. AAT deficiency is caused by mutations in SERPINA1, the gene that encodes the AAT protein, a serine protease inhibitor or serpin. The AAT protein is made in the liver and plays a crucial role in protecting lung function.
- Details
- Written by: Developer SM
- Hits: 439
Solid tumors are abnormal masses of tissue that occur in bones, muscles or other organs. Solid tumors may be cancerous or non-cancerous. Some of the most common solid tumor types occur in the breast, colon, bladder, prostate, and lung.

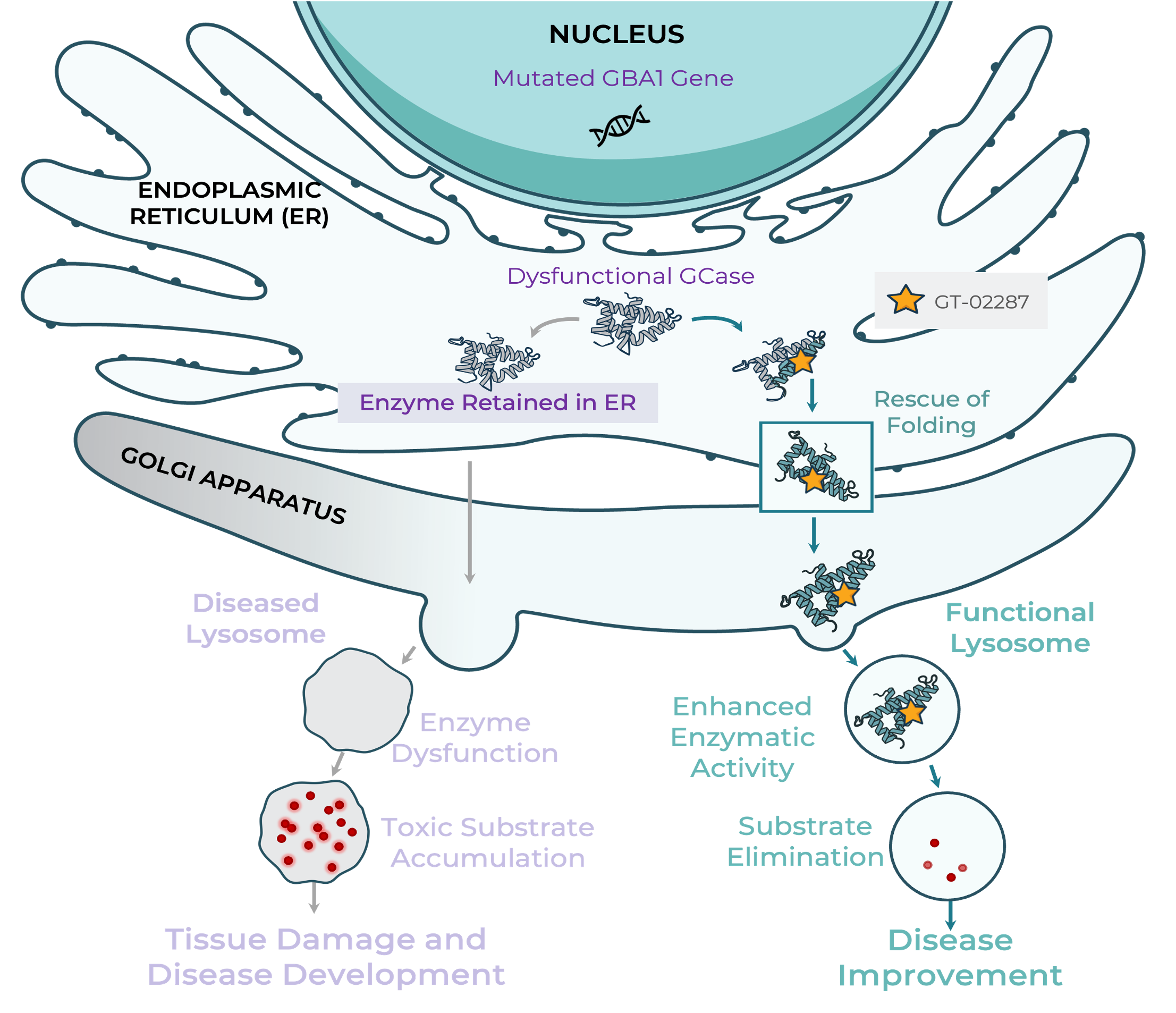
Gain Therapeutics’ lead drug candidate, GT-02287, is an allosteric regulator of GCase and is in development for the treatment of PD, with GBA1-PD as the lead indication. The orally administered, brain-penetrant small molecule restores the function of GCase. In preclinical models of PD, GT-02287 restored GCase enzymatic function, reduced cellular stress in the endoplasmic reticulum, improved lysosomal function and mitochondrial function, reduced toxic glycosphingolipids, aggregated α-synuclein, neuroinflammation and neuronal death, increased dopamine levels and improved motor function. Additionally, GT-02287 significantly reduced plasma neurofilament light chain (NfL) levels, an emerging biomarker for neurodegeneration.
Gain is conducting a Phase 1 clinical trial of GT-02287 in healthy volunteers with expected completion in the first half of 2024 and plans to initiate a patient cohort in that clinical trial in the third quarter of 2024.
More information about clinical trials can be found here